38 Development Of Atomic Theory Worksheet Answer Key
Early Ideas about Matter | Chemistry - Visionlearning Modern atomic theory An eighteenth-century chemistry bench. Priestley, Lavoisier, and others had laid the foundations of the field of chemistry. Their experiments showed that some substances could combine with others to form new materials, other substances could be broken apart to form simpler ones, and a few key "elements" could not be broken down any further. study.com › academy › lessonUnderstanding the Health Continuum: A Guide for Nurses ... Sep 20, 2021 · The health continuum is a tool used by nurses to educate patients on the trajectory of their health. Explore the illness-wellness continuum and examples of it, the 6 components of personal health ...
Atomic structure teaching resources - the science teacher GCSE worksheet on atomic structure key words. Key words used to describe atomic structure are written on the board. Students come up with a list of questions that their partner must answer by using the key words written on the slide. This activity promotes good discussion between students and supports meaning making of key terms.

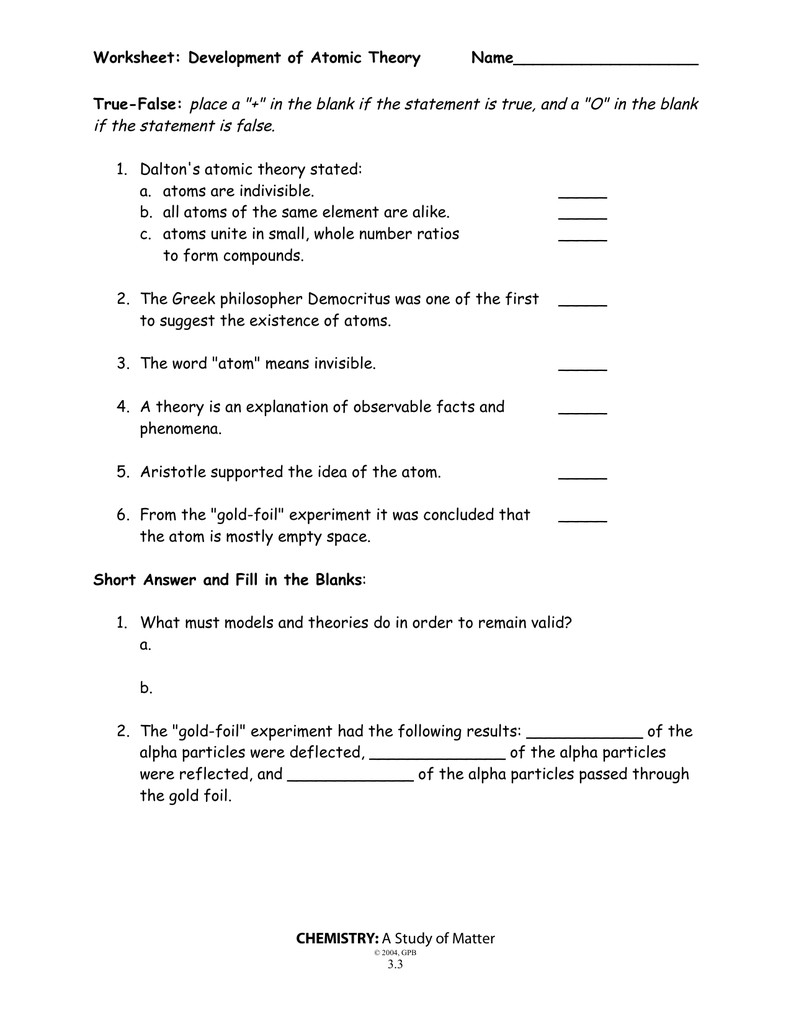
Development of atomic theory worksheet answer key
en.wikipedia.org › wiki › LightLight - Wikipedia Light or visible light is electromagnetic radiation within the portion of the electromagnetic spectrum that is perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750-420 terahertz, between the infrared (with longer wavelengths) and the ultraviolet (with shorter wavelengths). 1 The Development of Atomic Theory - Madison County Schools What scientists helped to develop atomic theory? • What part of atoms did Thomson discover? ... of positively charged particles at a very thin sheet of gold.6 pages 3.2 Determining Empirical and Molecular Formulas – Chemistry 2.1 Early Ideas in Atomic Theory. 2.2 Evolution of Atomic Theory. 2.3 Atomic Structure and Symbolism. 2.4 Chemical Formulas. 2.5 The Periodic Table. 2.6 Molecular and Ionic Compounds . 2.7 Chemical Nomenclature. Chapter 3. Composition of Substances and Solutions. Introduction. 3.1 Formula Mass and the Mole Concept. 3.2 Determining Empirical and Molecular Formulas. …
Development of atomic theory worksheet answer key. opentextbc.ca › chemistry › chapter2.5 The Periodic Table – Chemistry The number in square brackets is the atomic mass number (and approximate atomic mass) of the most stable isotope of that element. Key Concepts and Summary The discovery of the periodic recurrence of similar properties among the elements led to the formulation of the periodic table, in which the elements are arranged in order of increasing ... opentextbc.ca › chemistry › chapter15.2 Lewis Acids and Bases – Chemistry Key Concepts and Summary G.N. Lewis proposed a definition for acids and bases that relies on an atom’s or molecule’s ability to accept or donate electron pairs. A Lewis acid is a species that can accept an electron pair, whereas a Lewis base has an electron pair available for donation to a Lewis acid. Principles of Growth and Development - Video & Lesson ... 02.09.2021 · The principles of growth and development describe how the process of growth takes place. In this lesson, discover the three universal principles: cephalocaudal, proximodistal, and orthogenetic ... spadesman.blogspot.com › 2021 › 10Moles Gizmo Answer Key Pdf - spadesman.blogspot.com Oct 07, 2021 · Stoichiometry Gizmo Answer Key Pdf. Understand the definition of a mole and determine the Avogadro constant by adding atoms or formula units to a balance until the mass in grams is equal to the atomic or formula mass.
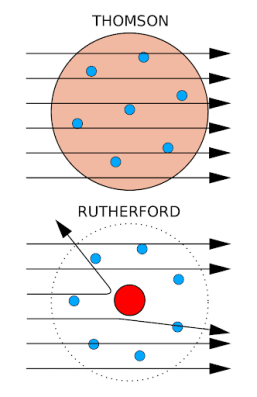
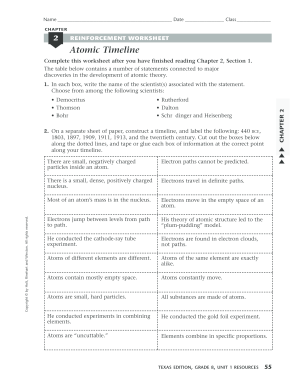
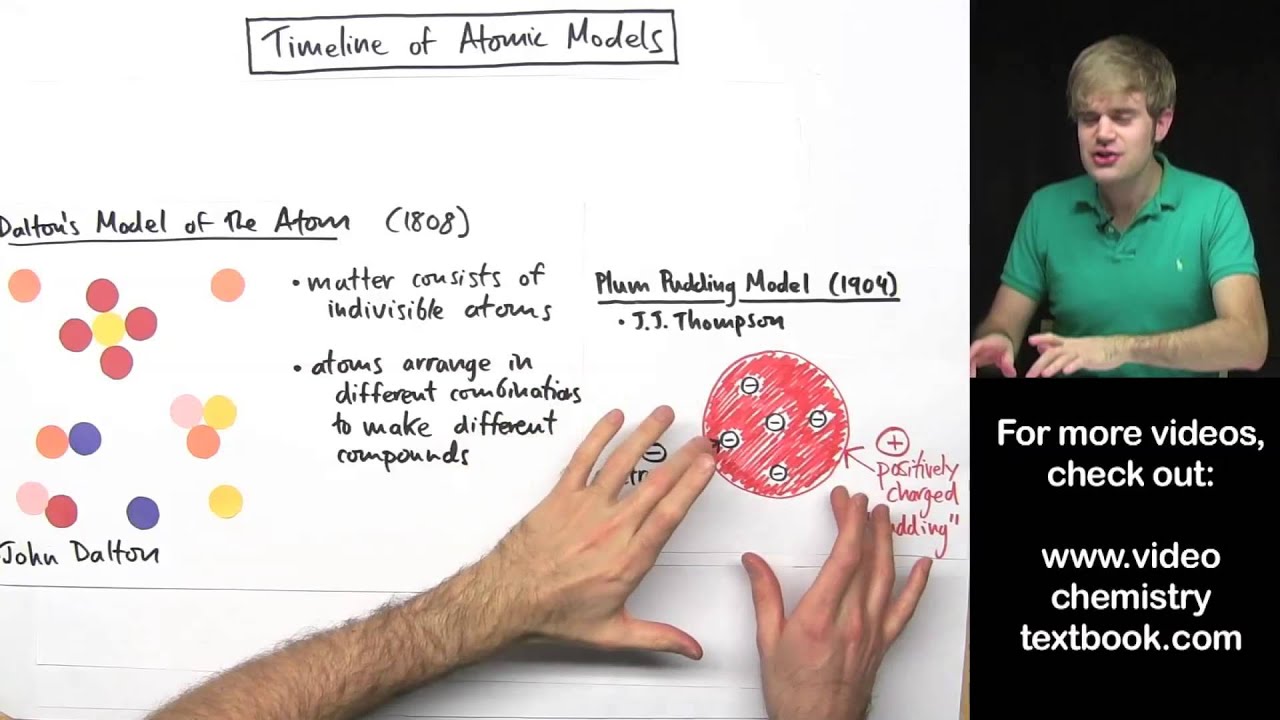
Development of the Atomic Theory - W. MACK LYON MIDDLE ... Dalton, shown in Figure 2, published his own atomic theory in 1803. ... a beam of small, positively charged particles at a thin sheet of gold foil.19 pages gizmos.explorelearning.com › indexElement Builder Gizmo : Lesson Info - ExploreLearning Use protons, neutrons, and electrons to build elements. As the number of protons, neutrons, and electrons changes, information such as the name and symbol of the element, the Z, N, and A numbers, the electron dot diagram, and the group and period from the periodic table are shown. Each element is classified as a metal, metalloid, or nonmetal, and its state at room temperature is also given. atomicdesign.bradfrost.com › chapter-2Atomic Design Methodology | Atomic Design by Brad Frost Atomic design is a helpful design and development methodology, but essentially it’s merely a mental model for constructing a UI. By now you may be wondering how you make atomic design happen. Well, fear not, dear reader, because the rest of the book focuses on tools and processes to make your atomic design dreams come true. 1 Development of the Atomic Theory Ernest Rutherford decided to shoot a beam of tiny, posi- tively charged particles at a thin sheet of gold foil. Rutherford guessed that atoms are soft blobs of ...8 pages
3.2 Determining Empirical and Molecular Formulas – Chemistry 2.1 Early Ideas in Atomic Theory. 2.2 Evolution of Atomic Theory. 2.3 Atomic Structure and Symbolism. 2.4 Chemical Formulas. 2.5 The Periodic Table. 2.6 Molecular and Ionic Compounds . 2.7 Chemical Nomenclature. Chapter 3. Composition of Substances and Solutions. Introduction. 3.1 Formula Mass and the Mole Concept. 3.2 Determining Empirical and Molecular Formulas. … 1 The Development of Atomic Theory - Madison County Schools What scientists helped to develop atomic theory? • What part of atoms did Thomson discover? ... of positively charged particles at a very thin sheet of gold.6 pages en.wikipedia.org › wiki › LightLight - Wikipedia Light or visible light is electromagnetic radiation within the portion of the electromagnetic spectrum that is perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750-420 terahertz, between the infrared (with longer wavelengths) and the ultraviolet (with shorter wavelengths).





















0 Response to "38 Development Of Atomic Theory Worksheet Answer Key"
Post a Comment