40 Mole To Mole Worksheet
DOC Stoichiometry Worksheet - Socorro Independent School District Stoichiometry Worksheet. Mole-Mole Stoichiometry Worksheet. 1. Given the following equation: 2 C4H10 + 13 O2 ---> 8 CO2 + 10 H2O. Show what the following molar ratios should be. a. C4H10 / O2 2:13 b. O2 / CO2 c. O2 / H2O d. C4H10 / CO2 e. PDF 2 3 2 3 2 1 2H3O2 3 2 ? f.u. NaCl = 3.55 mol NaCl 6.02 H ... Title: Microsoft Word - 7-06a,b Mole Problems Wkst-Key.doc Author: Brent White Created Date: 6/23/2005 8:48:34 PM
PDF Mole Conv WS Key - Anoka-Hennepin School District 11 Created Date: 2/23/2015 4:14:14 PM

Mole to mole worksheet
Stoichiometry Mole To Mole Worksheets - Kiddy Math Stoichiometry Mole To Mole - Displaying top 8 worksheets found for this concept. Some of the worksheets for this concept are Work on moles and stoichiometry, Schoolnotes, Mole calculation work, Chemistry as fun and games, Skills work problem solving, Stoichiometry work 1 b 27, Name block stoichiometry 2 mass relationships, Moles work answers. PDF Stoichiometry Worksheet #2 (mole-mass, mass-mole problems) 1 mol N 2 = 5.6x10-g N 2 2. K 3 PO 4 + Al(NO 3) 3 → 3KNO 3 + AlPO 4 a. What is the mass of potassium nitrate that is produced when 2.04 moles of potassium phosphate react? 2.04 mol K 3 PO 4 3 mol KNO 3 101.1 g KNO 3 1 mol K 3 PO 4 1 mol KNO 3 = 619g KNO 3 b. If 5.80g of aluminum phosphate are formed, how many moles of aluminum nitrate reacted? 5.80g AlPO 4 1 mol AlPO 4 1 mol Al(NO 3) PDF Mole To Mole Wksht Key20130206141658866 STOICHIOMETRY WORKSHEET (MOLE-MOLE) I. Magnesium reacts with hydrochloric acid according to the following balanced chemical equation: Mg (s) + 2 HCI (aq) MgC12 (aq) + Ha (g) If two moles of h drochloric acid react with excess magnesium, how many moles of hy rogen gas will be produced? 2 mole Hd I mole H 2 H2 HCI 2.
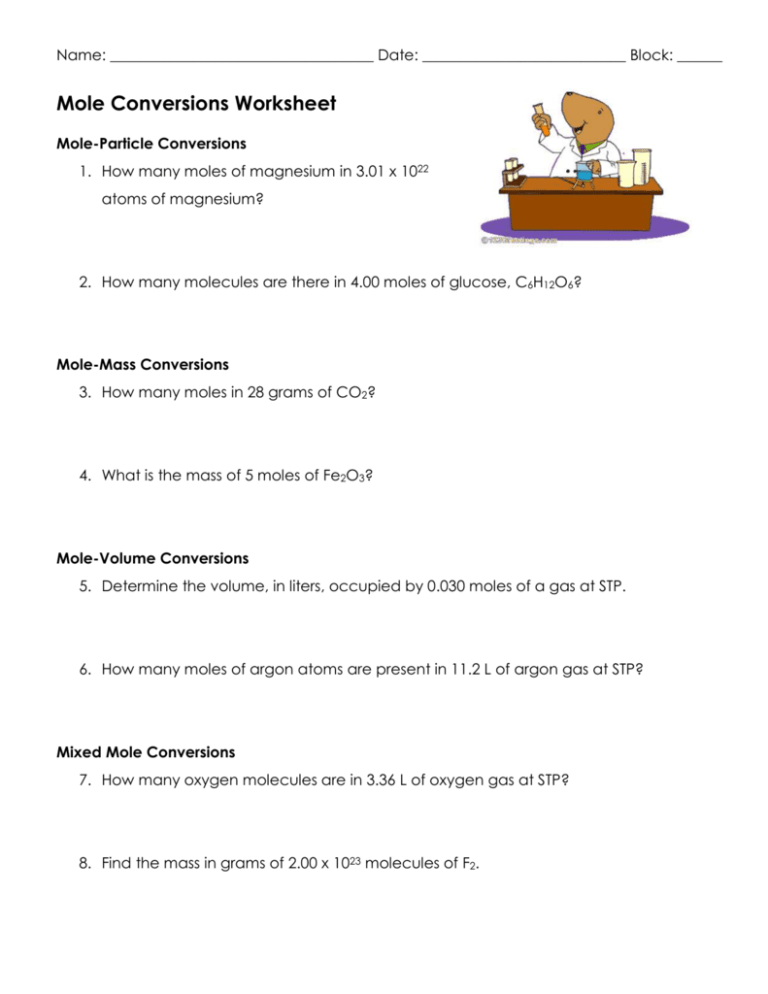
Mole to mole worksheet. PDF Skills Worksheet Problem Solving over and over again, chemists refer to it as a mole (abbreviated mol). 6.022 1023 objects is called a mole, just as you call 12 objects a dozen. Look again at how these quantities are related. 55.847 g of iron 6.022 1023 iron atoms 1 mol of iron 32.066 g of sulfur 6.022 1023 sulfur atoms 1 mol of sulfur General Plan for Converting Mass, Amount, DOCX Mole Conversions Worksheet - Anoka-Hennepin School District 11 3. Determine the number of molecules of 14 g of nitrogen dioxide (NO2). 4. Find the mass, in grams, of 1.00 x 1023 molecules of N2. 5. Aspartame is an artificial sweetener that is 160 times sweeter than sucrose (table sugar) when dissolved in water. It is marketed by G.D. Searle as. DOC Stoichiometry: Mole to Mass Problems How many moles of water (H2O) are produced when 25.0 grams of C2H2 burns completely? 0.960 moles H20. 4. 3 Mg + 1 Fe2O3 ( 2 Fe + 3 MgO. How many moles of iron, Fe, are produced with 25.0 grams of magnesium, Mg? 0.686 mole Fe. 5. Laughing gas (nitrous oxide, N2O) is sometimes used as an anesthetic in dentistry. DOC The Mega Mole Worksheet - welcome to Ms. Botticelli's ... The Mega Mole Worksheet #1-10 Convert to Moles 12.04 x 1023 atoms He. 3.01 x 1023 atoms Cu. 3.612 x 1023 atoms Fe. 100 atoms Ar. 1 atom S. 24 grams S
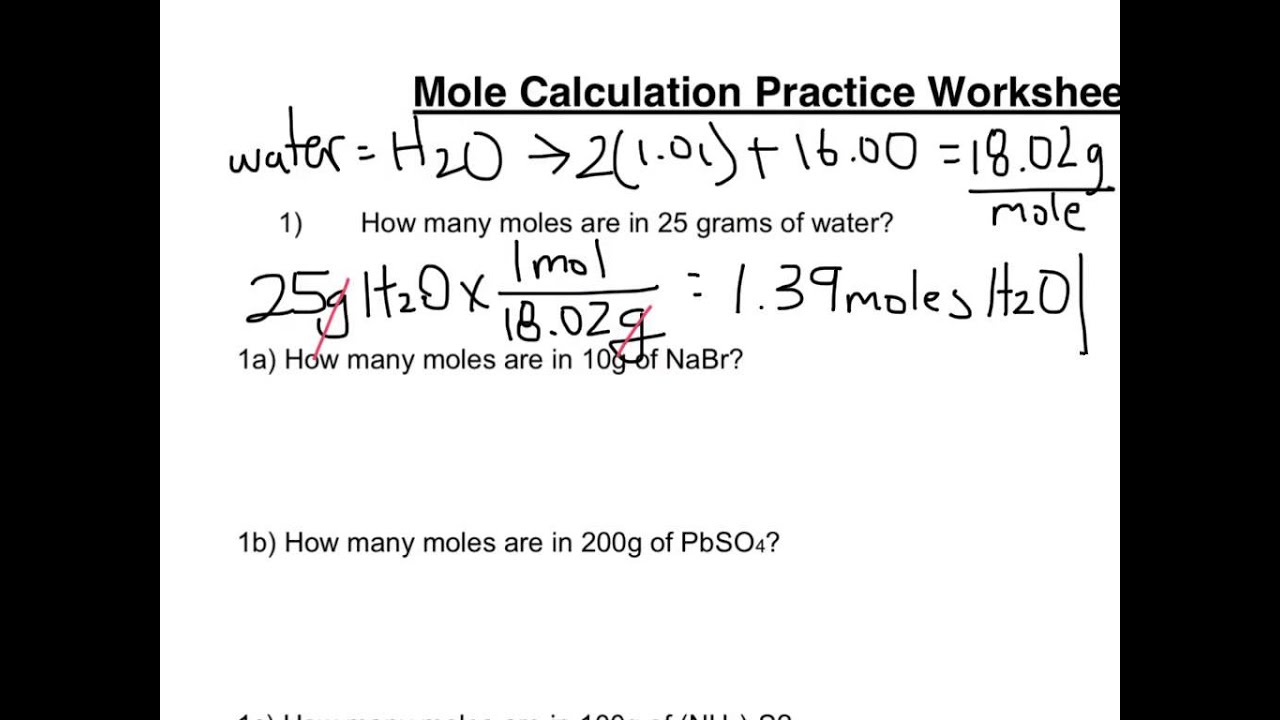
Worksheet - Mole Conversions - Quia 1. How many moles are in 122.5 g of magnesium hydroxide? 122.5 g Mg(OH)2 x 1 mol Mg(OH)2 = 2.100 mol Mg(OH)2. 58.32 g Mg(OH)2. 2. How many moles are in 2.45 g of hydrogen gas (H2)? 2.45 g H2 x 1 mol H2 = 1.21 mol H2. 2.02 g H2. 3. How many grams are in 6.3 moles of ammonium chloride? 6.3 mol NH4Cl x 53.50 g NH4Cl = 340 g NH4Cl PDF Mole Calculation Worksheet - Everett Community College Mole Calculation Worksheet W 340 Everett Community College Tutoring Center Student Support Services Program 1) How many moles are in 40.0 grams of water? 2) How many grams are in 3.7 moles of Na 2 O? 3) How many atoms are in 14 moles of cadmium? 4) How many moles are in 4.3 x 1022 molecules of H 3 PO 4? 5) How many molecules are in 48.0 grams of NaOH? PDF Moles Worksheet - Science Classroom Teacher Resources Moles Worksheet (Solutions) 1) Define "mole". 6.02 x 1023 of anything, usually atoms or molecules. 2) How many moles are present in 34 grams of Cu(OH)2? 0.35 moles 3) How many moles are present in 2.45 x 1023 molecules of CH 4? 0.41 moles 4) How many grams are there in 3.4 x 1024 molecules of NH 3? 96 grams 5) How much does 4.2 moles of Ca(NO3)2 weigh? PDF Mole Calculation Worksheet - Science Classroom Teacher ... Mole Calculation Worksheet - Answer Key 1) How many moles are in 15 grams of lithium? 0.46 moles 2) How many grams are in 2.4 moles of sulfur? 77.0 grams 3) How many moles are in 22 grams of argon? 0.55 moles 4) How many grams are in 88.1 moles of magnesium? 2141 grams 5) How many moles are in 2.3 grams of phosphorus? 0.074 moles
rosestyle.de Concept Review. MOLE WORKSHEET #2 Convert to moles: 1. worksheet_-_mole_ratio_introduction_key. OR. This worksheet consists of 13 problems. As well as Calorimetry Worksheet 1) If 0. Subject: Chemistry. 0154 mole of product is formed. #chemistry, #mole, #moleday, #avogadro. 4278g. chemistry unit 8 worksheet 1 mole relationships answers. PDF Mole Calculation Worksheet - Brookside High School 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 L of a gas at STP ( You do not need to worry about this yet ) Each definition can be written as a set of two conversion factors. DOC Stoichiometry: Mole-Mole Problems 2KClO3 → 2KCl + 3O2. How many moles of oxygen are produced by the decomposition of 6.0 moles of potassium chlorate? 9.0 moles of oxygen. Zn + 2HCl → ZnCl2 + H2. How many moles of hydrogen are produced from the reaction of 3.0 moles of zinc with an excess of hydrochloric acid? 3.0 moles of hydrogen. 4. DOC Worksheet for Basic Stoichiometry Worksheet for Basic Stoichiometry. Part 1: Mole ←→ Mass Conversions. Convert the following number of moles of chemical into its corresponding mass in grams. 1. 0.436 moles of ammonium chloride. 2. 2.360 moles of lead (II) oxide. 3. 0.031 moles of aluminum iodide. 4. 1.077 moles of magnesium phosphate. 5. 0.50 moles of calcium nitrate
Mole Conversion Worksheet and Activity ⋆ iTeachly.com The number of moles of 99.4 grams of NaCl. Expert Level (hint you must use both equations) The molarity when 54.8 grams of lithium sulfate are dissolved to make 250 mL of solution. The molarity when 99.1 grams of (NH 4)2SO4 are dissolved to make 0.5 L of solution.
Stoichiometry Worksheet 1 Mole To Mole Answer Key ... Worksheet Stoichiometry Worksheet 1 Mole To Mole Answer Key by Amanda on July 6, 2021 Leave a Comment 1 mole 602 x 1023 particles 1 mole molar mass could be atomic mass from periodic table or molecular mass 1 mole 224 L of a gas at STP You do not need to worry about this yet Each definition can be written as a set of two conversion factors.
14 Best Images of Mole To Mole Conversions Worksheet ... Talking related with Mole to Mole Conversions Worksheet Answer Key, we have collected various similar images to inform you more. mole conversion worksheet answer key, mole conversion worksheet answer key and mole conversion worksheet answers are some main things we will show you based on the gallery title.
Mole To Mole Worksheet | Teachers Pay Teachers The Mole - Practice Worksheets with representative particles, volume, and mass, empirical formula calculations, molecular formula calculationsThis bundle includes 8 homework assignments that cover the following topics:Worksheet 1- Using the conversion: 1mole=6.02x10^23 Worksheet 2- Calculating Molar
Mole To Mole Stoichiometry Worksheet - worksheet Stoichiometry mole to mole displaying top 8 worksheets found for this concept. 1 mole 22 4 l of a gas at stp you do not need to worry about this yet each definition can be written as a set of two conversion factors. 1 mole molar mass g can be written as 1 mole or molar mass g molar mass g 1 mole.
PDF Grams/Moles Calculations Worksheet III 1) 30 grams of H 3PO 4 (phosphoric acid) 0.31 moles of H3PO 4 2) 25 grams of HF (hydrofluoric acid) 1.25 moles HF 3) 110 grams of NaHCO 3 (sodium bicarbonate or baking soda) 1.31 moles 4) 1.1 grams of FeCl 3 (iron (III) chloride) 0.0068 moles 5) 987 grams of Ra(OH) 2 (radium hydroxide) 3.80 moles 6) 564 grams of copper (copper) 0.11 moles
PDF CHEMISTRY: A Study of Matter - Weebly a. How many moles of oxygen react with 11 moles of C 3H8? ? mol O 2 = 11 mol C 3 H 8 × 5 mol O 2 1 mol C 3 H 8 = 55 mol O 2 b. How many moles of CO 2 are produced if 3.5 moles of water are produced? ? mol CO 2 = 3.5 mol H 2 O × 3 mol CO 2 4 mol H 2 O = 2.6 mol CO 2 5. ___O 2 + ___Fe ___Fe 2O3 a. Fill in the following word equation--_____ moles of oxygen gas react
PDF Mole Conversions Practice Worksheet with Key Use a separate piece of paper, show all work, and circle your final answer. (Attach this sheet to your work). Set A: One Step Problems: Convert to moles: Convert to mass in grams: 10.0 moles Na 11. 12. 2.20 moles Sn 13. 5.00 moles Ag 14. 3.0 x 104 moles Au 15. 1.00 x 10-7 moles B Convert to number of atoms: 4.
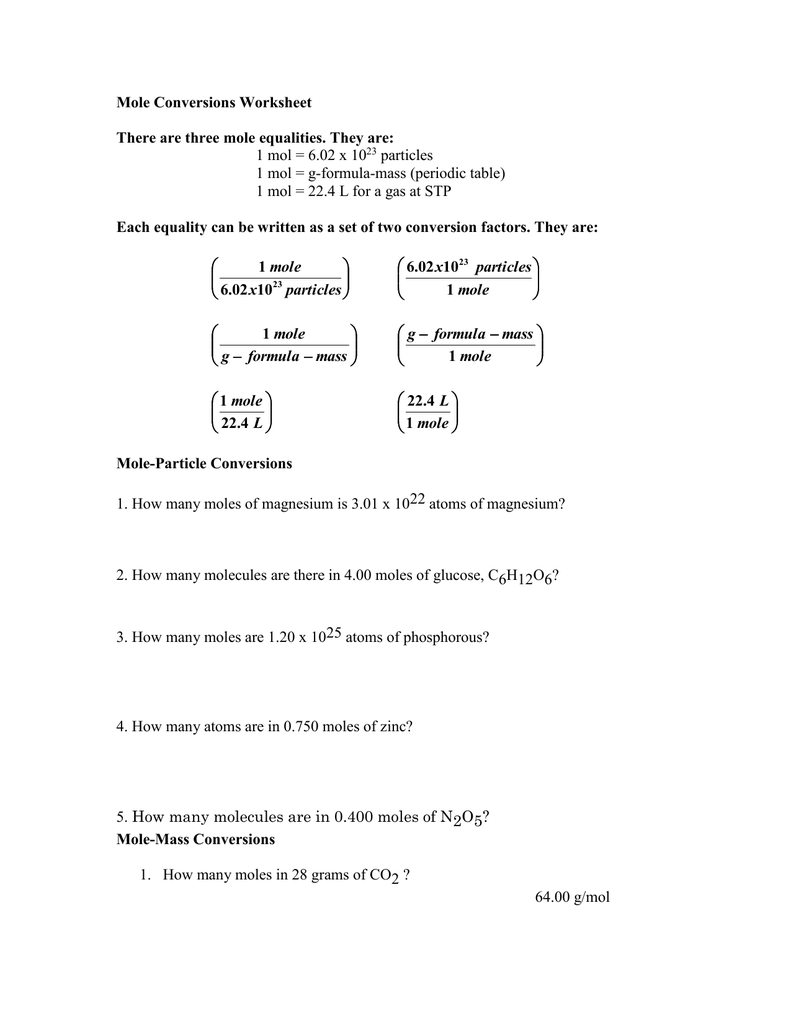
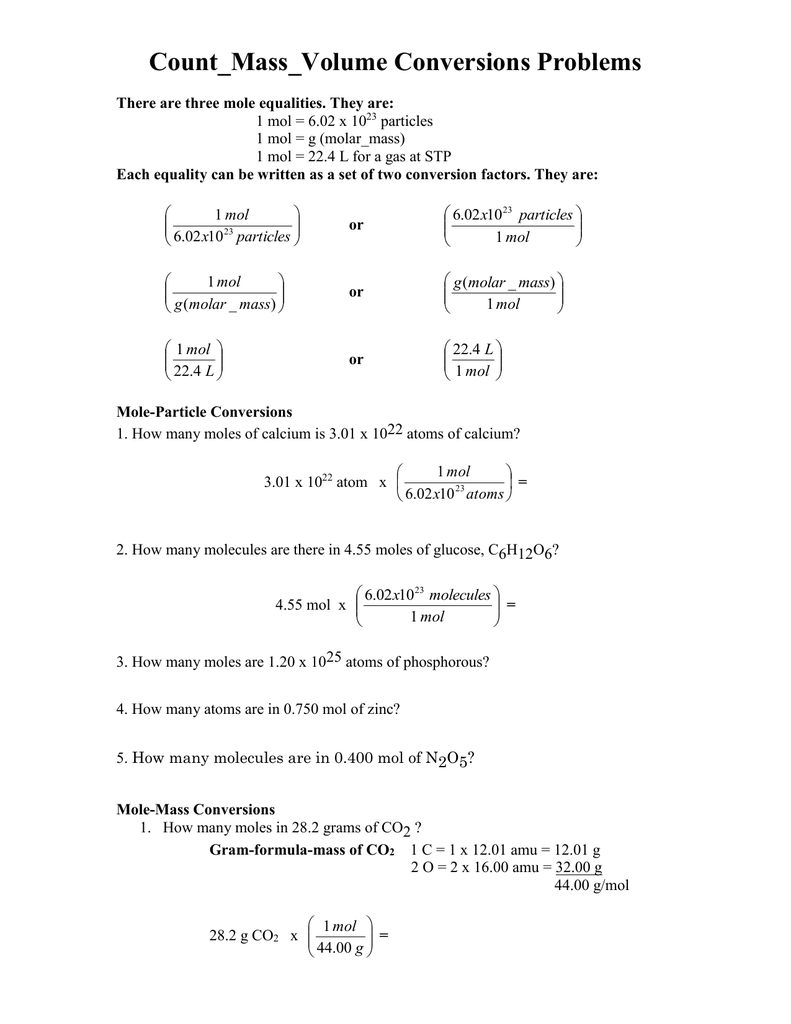
PDF Mole to Grams, Grams to Moles Conversions Worksheet Mole to Grams, Grams to Moles Conversions Worksheet What are the molecular weights of the following compounds? 1) NaOH 2) H 3PO 4 3) H 2O 4) Mn 2Se 7 5) MgCl 2 6) (NH 4) 2SO 4 There are three definitions (equalities) of mole. They are: 1 mole = 6.02 x 1023 particles 1 mole = molar mass (could be atomic mass from periodic table or molecular mass)
DOC Stoichiometry Worksheet 1 (Mole-Mole Conversions) [HNO3] = # mols ÷ volume So mols HNO3 = 12.0 M x 0.01 L = 0.12 mol HNO3. 0.12 mol HNO3 1 mol Cu 63.5 g Cu 4 mol HNO3 1 mol Cu = 1.91 g Cu (Limiting Reactant and % Yield) Identify the limiting reactant when 1.22g of O2 reacts with 1.05g of H2 to produce water. O2 is limitting
DOC Mole Ratio Worksheet - Oswego Community Unit School ... Mole Ratio Worksheet. 1) Balance this equation: N2 + H2 ---> NH3, write the following molar ratios: N2 / H2 N2 / NH3 H2 / NH3. 2) Balance this equation: H2 + S ---> H2S, write the following molar ratios: H2 / H2S H2 / S H2S / S. 3) Write and balance the equation for the synthesis of water. Then answer the following questions.
PDF Mole To Mole Wksht Key20130206141658866 STOICHIOMETRY WORKSHEET (MOLE-MOLE) I. Magnesium reacts with hydrochloric acid according to the following balanced chemical equation: Mg (s) + 2 HCI (aq) MgC12 (aq) + Ha (g) If two moles of h drochloric acid react with excess magnesium, how many moles of hy rogen gas will be produced? 2 mole Hd I mole H 2 H2 HCI 2.
PDF Stoichiometry Worksheet #2 (mole-mass, mass-mole problems) 1 mol N 2 = 5.6x10-g N 2 2. K 3 PO 4 + Al(NO 3) 3 → 3KNO 3 + AlPO 4 a. What is the mass of potassium nitrate that is produced when 2.04 moles of potassium phosphate react? 2.04 mol K 3 PO 4 3 mol KNO 3 101.1 g KNO 3 1 mol K 3 PO 4 1 mol KNO 3 = 619g KNO 3 b. If 5.80g of aluminum phosphate are formed, how many moles of aluminum nitrate reacted? 5.80g AlPO 4 1 mol AlPO 4 1 mol Al(NO 3)
Stoichiometry Mole To Mole Worksheets - Kiddy Math Stoichiometry Mole To Mole - Displaying top 8 worksheets found for this concept. Some of the worksheets for this concept are Work on moles and stoichiometry, Schoolnotes, Mole calculation work, Chemistry as fun and games, Skills work problem solving, Stoichiometry work 1 b 27, Name block stoichiometry 2 mass relationships, Moles work answers.





























0 Response to "40 Mole To Mole Worksheet"
Post a Comment