41 chemistry average atomic mass worksheet answers
Practice - Average Atomic Mass Worksheet 1.0 - Answer Key Bundle - Average Atomic Mass Practice Worksheet 1.0 & Answer Key Save 10% off regularly priced items with this bundle!!The Chemistry Teacher WebsiteThe Chemistry Teacher on YouTube 2 Products $2.23 $2.48 Save $0.25 View Bundle Bundle - Average Atomic Mass Practice Worksheets 1.0, 1.1, & 2.0 + Answer Keys Calculating Average Atomic Mass Worksheet | Aurumscience.com. This worksheet will show students how these numbers are calculated, and help them understand why the atomic mass of oxygen is 15.99 AMU instead of simply 16 AMU. Essential Concepts: Isotopes, average atomic mass, isotope abundance, atomic mass units. Answer key: Included in the chemistry instructor resources subscription. Click here for details.
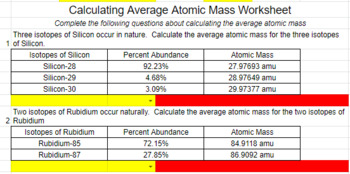
average_atomic_mass_worksheet_answers.pdf - I: Oaks... View Homework Help - average_atomic_mass_worksheet_answers.pdf from CHEM 101 at Oaks Christian School. I: Oaks Christian ' Chemistry H Name: [:1 \ 1 $5M. Section: Date: Jnit 2 - Atomic
Chemistry average atomic mass worksheet answers
Isotopes & Relative Atomic Mass (solutions, examples, videos) An atom of carbon-12 is taken to have a mass of 12 atomic mass unit (amu). Since one carbon-12 atom has 6 proton and 6 neutron, mass of one proton (neutron) = mass of one carbon-12 atom = 1 amu (atomic mass unit) Atomic Mass Units. Average Atomic Mass and definition of atomic mass unit. Show Video Lesson PDF Answers Key for Unit Worksheets - livingston.org The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24 Mg (78.70%), 25Mg (10.13%), and 26Mg(11.7%). 'The average atomic mass of the three isotopes is 24.3050 amu. Isotopes & Calculating Average Atomic Mass - AACT In this simulation, students first learn how the average atomic mass is determined through a tutorial based on the isotope abundance for Carbon. Students will then interact within a workspace where they will select the number of isotopes, the mass of each isotope as well as their abundancies in order to successfully build a mystery element.
Chemistry average atomic mass worksheet answers. How to Calculate Average Atomic Mass | Chemistry | Study.com How to Calculate Average Atomic Mass: Example 1. Calculate the average atomic mass of boron given that 19.8% of its naturally occurring atoms have a mass of 10.013 amu and 80.2% have a mass of 11. ... Assignment Essays - Best Custom Writing Services Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply. PDF Isotope Practice Worksheet - Chemistry Answer: The atomic mass of boron is 10.811; therefore, boron-11 is more abundant because the mass number is closer to the atomic mass. 8. Lithium-6 is 4% abundant and lithium-7 is 96% abundant. What is the average mass of lithium? Answer: 6.96 amu 9. Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine. Answer ... average atomic mass practice ws KEY.docx - Name _ Period Find the average atomic mass of Element X. (13.2amu) (60) + (14.1amu) (40) = 13.56amu 100 ( 13. 2 amu ) ( 60 ) + ( 14.1 amu ) ( 40 ) = 13.56amu 100 2. Two isotopes are known for Element Y. 35.0% of all the atoms of Element Y have an atomic mass of 29.7 amu. 65.0% of the isotopes have an atomic mass of 32.0amu.
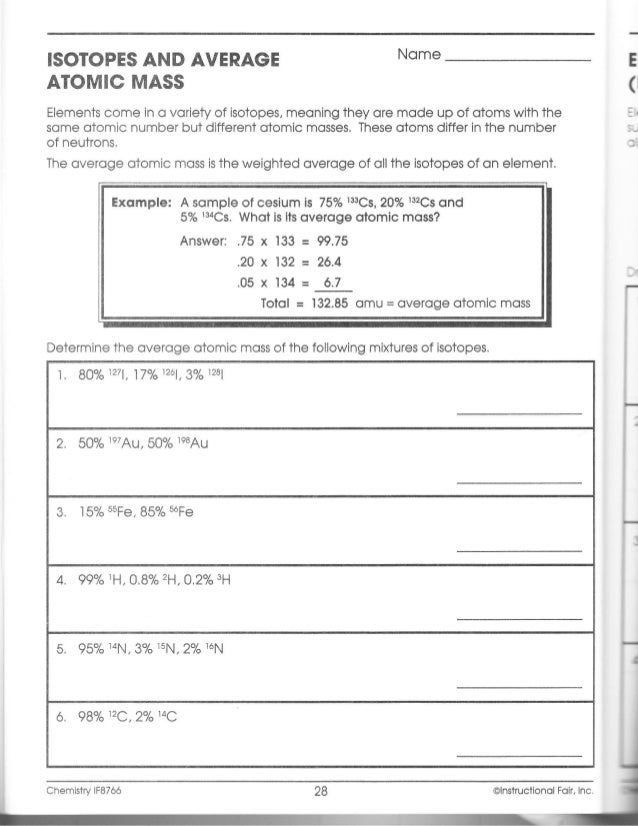
PDF KMBT 654-20131024112244 - Berger's Chemistry Class What is its average atomic mass? Answer: .75 x 133 = 99.75 .20 x 132 = 26.4 .05 x 134 = Total = 132.85 amu = average atomic mass Determine the average atomic mass of the following mixtures of isotopes. l. IZC 2. 500/0 3. 150/0 4. Il-I, 0.8%4-1, 0.2% 0.01b 5. OMS 13, B 0t46 6. 980/0 Chemistry IF87ó6 PDF Average Atomic Mass - Mr. Sault's Classroom a. Which isotope has an atomic mass closest to the average atomic mass listed on the periodic table? 24.mg b. Give a mathematical reason for your answer to part a. 24 Mg is the most common isotope and is thus most heavily "weighted" in the equation for average atomic mass. 17. Boron has two naturally occurring isotopes: boron-10 and boron-11. Quiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element? Atomic Structure Worksheet - Washoe County School District The atomic number gives the “identity “of an element as well as its location on the Periodic Table. No two different elements will have the atomic number. The of an element is the average mass of an element’s naturally occurring atoms, or isotopes, taking into account the of each isotope.
DOC Chemistry Worksheet - Humble Independent School District Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine. Atomic Number And Atomic Mass Worksheets - K12 Workbook Displaying all worksheets related to - Atomic Number And Atomic Mass. Worksheets are Chemistry work atomic number and mass number, Atomic mass and atomic number work, Atomic structure work, Teacher workbooks, Example exercise atomic mass and avogadros number, Protons neutrons and electrons practice work, Preview, Basic atomic structure work key 1. Average Atomic Mass Practice Problems Quiz - Quizizz Calculate the average atomic mass of an element with the follow isotope information: 4.35% have a mass of 49.9461 amu, 83.79% have amass of 51.9405 amu, 9.50% have a mass of 52.9407 amu, and 2.36% have a mass of 53.9389 amu. answer choices 51.99 amu 52.19 amu 53.45 amu 17.33 amu Question 4 900 seconds PDF AVG Atomic Mass WS Key - livingston.org Subject: Image Created Date: 9/28/2012 8:47:13 AM
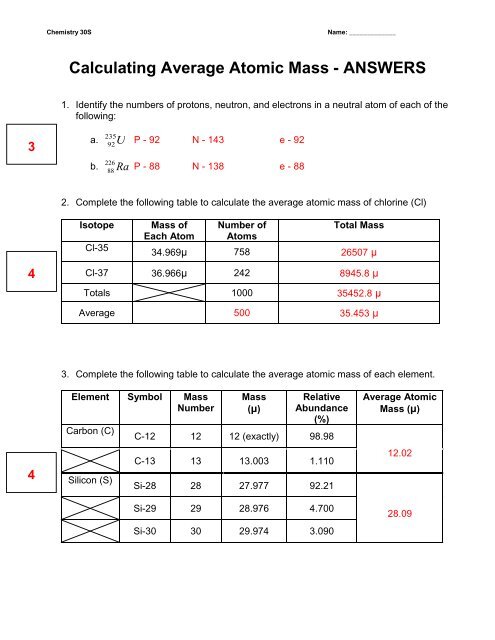
Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet Find the average atomic mass for Cl is 75.78% of Cl atoms are 35Cl with a mass of 34.96885271 amu and 24.22% are 37Cl with a mass of 36.96590260 amu. (75.78 x 34.96885271) + (24.22 x 36.965902060) / 100; 2649.939658 + 895.314161 / 100 = 35.45 = Cl
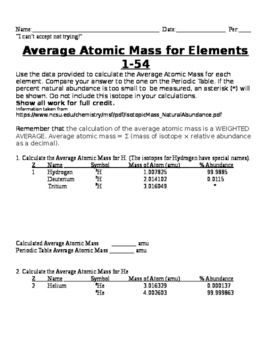
Calculating Average Atomic Mass Worksheet Name 1. The term "average atomic mass" is a _________________________average, and so is calculated differently from a "normal" average. Explain how this type of average is calculated. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are: 69.2% for mass of 62.93u
DOC Chemistry Worksheet - Forestville Central High School Average Atomic Mass. Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average ...
20 atomic Mass and Number Worksheet | Worksheet From Home Atomic Mass Atomic Number Worksheet 1 November 6 chemistry atomic number and mass number worksheet answers, atomic mass number worksheet, atomic number and mass number worksheet tes, atomic mass and atomic number worksheet pdf answers, average atomic mass and isotopes worksheet answers, via: pinterest.com Numbering Worksheets for Kids.
PDF Average Atomic Mass Practice Problems - scott.kyschools.us 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and
Chapter 6 – Quantities in Chemical Reactions – Chemistry The mass of 1 mol of molecules (or formula units) in grams is numerically equivalent to the mass of one molecule (or formula unit) in atomic mass units. For example, a single molecule of O 2 has a mass of 32.00 u (the sum of 2 oxygen atoms), and 1 mol of O 2 molecules has a mass of 32.00 g.
Atomic Mass Atomic Number Worksheets - K12 Workbook 1. Chemistry Worksheet, Atomic Number and Mass Number 2. Atomic Structure Review Worksheet Answers 3. Mayfield High School 4. Chemistry Atomic Number And Mass Number 5. Atomic Structure Periodic Table Worksheet Answers 6. CHAPTER 4: Chemical patterns Worksheet 4.2 Science Quest ... 7. Atomic Structure Chapter 4 Worksheet Answers 8.
More Average Atomic Mass Worksheet Answers And Average Atomic Mass Worksheet Answers solutions for you to be successful. Average atomic mass worksheet answer key atomic structure worksheet. The average atomic mass of an isotope is also based on many factors. The other isotope 65Cu has an abundance of 3091. Average Atomic Mass How are the masses on the periodic table determined.
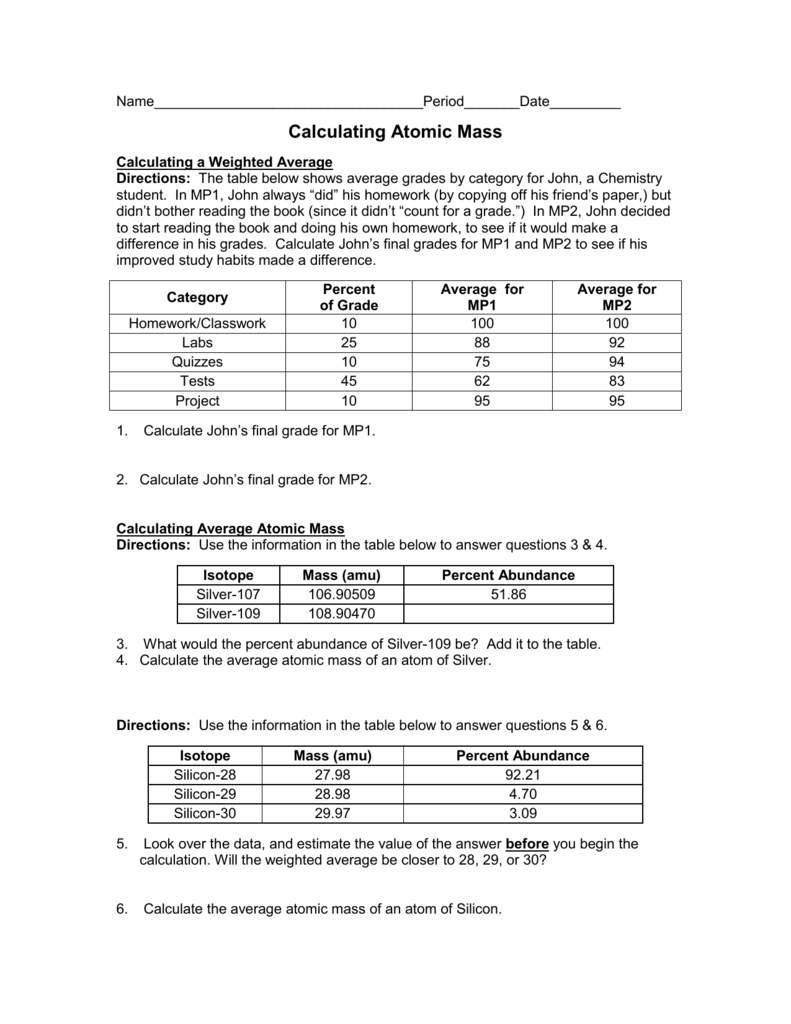
PDF NAME Average Atomic Mass Worksheet: show all work. and 24.47 percent 37Cl (mass = 36.966 amu). Calculate the average atomic mass. 35.46 amu 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63 ...
Honors Chemistry Worksheet - Atomic Structure The number of neutrons in an isotope may be determined by subtracting the number of protons (atomic number) from the mass number. #n0 = A - Z. If working with the element, the atomic mass may be rounded to a whole number and used as a mass number. 9. An atomic nucleus contains 6 protons and 6 neutrons. About the nucleus move 2 electrons in the ...
Calculating Average Atomic Mass Worksheet - Name ___Lisa ... - StuDocu The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit. 69/100 x 62 = 43. 30/100 x 64 = 19. ggghvhvhvhvhhv= 63-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a ...
Calculating Average Atomic Mass Practice | Chemistry Practice Problems ... Calculating Average Atomic Mass High School Chemistry Skills Practice 1. Carbon has three isotopes, namely Carbon-12, Carbon-13, and Carbon-14. C-12 has a mass of 12.000 amu and is 98.89%...
Chemistry Lesson: Average Atomic Mass Calculations average atomic mass = ∑ (relative abundance x mass of isotope) Remember that ∑ is the symbol for sum. In other words, we will take the sum of the relative abundance of each isotope multipled by its mass. Example Neon has three naturally occuring isotopes. Remember that mass number is not the same as the atomic mass or isotopic mass!
Calculating Average Atomic Mass Worksheet - Name ... - StuDocu The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit.-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a mass of 33. Show ALL work for full credit.
Chemistry Quizzes | Study.com 2,000,000+ Questions and Answers ... Food Chemistry: Quiz & Worksheet for Kids . View Quiz. ... Isotopes and Average Atomic Mass . View Quiz. Predict the Formation, Charge, and Formulas of Ions ...
Isotopes & Calculating Average Atomic Mass - AACT In this simulation, students first learn how the average atomic mass is determined through a tutorial based on the isotope abundance for Carbon. Students will then interact within a workspace where they will select the number of isotopes, the mass of each isotope as well as their abundancies in order to successfully build a mystery element.
PDF Answers Key for Unit Worksheets - livingston.org The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24 Mg (78.70%), 25Mg (10.13%), and 26Mg(11.7%). 'The average atomic mass of the three isotopes is 24.3050 amu.
Isotopes & Relative Atomic Mass (solutions, examples, videos) An atom of carbon-12 is taken to have a mass of 12 atomic mass unit (amu). Since one carbon-12 atom has 6 proton and 6 neutron, mass of one proton (neutron) = mass of one carbon-12 atom = 1 amu (atomic mass unit) Atomic Mass Units. Average Atomic Mass and definition of atomic mass unit. Show Video Lesson































0 Response to "41 chemistry average atomic mass worksheet answers"
Post a Comment