43 more average atomic mass worksheet answers
› molecular-mass-calculationsMolecular Mass Calculations - ThoughtCo Mar 11, 2019 · Find the atomic mass for each element by using the mass given in the Periodic Table. Multiply the subscript (number of atoms) times the atomic mass of that element and add the masses of all of the elements in the molecule to get the molecular mass. For example, multiple the subscript 12 times the atomic mass of carbon (C). wou.edu › chemistry › coursesChapter 6 – Quantities in Chemical Reactions – Chemistry The mass of 1 mol of molecules (or formula units) in grams is numerically equivalent to the mass of one molecule (or formula unit) in atomic mass units. For example, a single molecule of O 2 has a mass of 32.00 u (the sum of 2 oxygen atoms), and 1 mol of O 2 molecules has a mass of 32.00 g.
en.wikipedia.org › wiki › Atomic_numberAtomic number - Wikipedia A little more than three-quarters of naturally occurring elements exist as a mixture of isotopes (see monoisotopic elements), and the average isotopic mass of an isotopic mixture for an element (called the relative atomic mass) in a defined environment on Earth, determines the element's standard atomic weight. Historically, it was these atomic ...

More average atomic mass worksheet answers
rlk.meerfarbiges.de › exploring-science-answersExploring science answers year 7 - Fit2future * Detailed Technician notes * All the answers to all the questions in the Student Book and. Exploring science 7 answers Chps 4 and 5 Atoms to Minerals Notes (PDF 7.08 MB) Chp 6: Rock Notes (PDF 2.18 MB) Chp 14: Weathering, Soils, and Mass Wasting Notes (PDF 7.53 MB) Chp 15 & 16: Surface water and Groundwater Notes (PDF 4.87 MB) Chapter 10 Plate ... › cms › lib6Basic Atomic Structure Worksheet Key - Neshaminy School District have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. The q of an element is the total number of protons and neutrons in the of atom. The mass number is used to calculate the number of O in one atom of an element. In nap.nationalacademies.org › read › 131657 Dimension 3: Disciplinary Core Ideas - Earth and Space ... Earth exchanges mass and energy with the rest of the solar system. It gains or loses energy through incoming solar radiation, thermal radiation to space, and gravitational forces exerted by the sun, moon, and planets. Earth gains mass from the impacts of meteoroids and comets and loses mass from the escape of gases into space.
More average atomic mass worksheet answers. byjus.com › maths › quartile-deviationQuartile Deviation - Definition, Formula and Examples - BYJUS Quartile deviation is one of the measures of dispersion.Before getting into a deeper understanding, let’s recall quartiles and how we can define them. Quartiles are the values that divide a list of numerical data into three-quarters, such as Q 1, Q 2 and Q 3. nap.nationalacademies.org › read › 131657 Dimension 3: Disciplinary Core Ideas - Earth and Space ... Earth exchanges mass and energy with the rest of the solar system. It gains or loses energy through incoming solar radiation, thermal radiation to space, and gravitational forces exerted by the sun, moon, and planets. Earth gains mass from the impacts of meteoroids and comets and loses mass from the escape of gases into space. › cms › lib6Basic Atomic Structure Worksheet Key - Neshaminy School District have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. The q of an element is the total number of protons and neutrons in the of atom. The mass number is used to calculate the number of O in one atom of an element. In rlk.meerfarbiges.de › exploring-science-answersExploring science answers year 7 - Fit2future * Detailed Technician notes * All the answers to all the questions in the Student Book and. Exploring science 7 answers Chps 4 and 5 Atoms to Minerals Notes (PDF 7.08 MB) Chp 6: Rock Notes (PDF 2.18 MB) Chp 14: Weathering, Soils, and Mass Wasting Notes (PDF 7.53 MB) Chp 15 & 16: Surface water and Groundwater Notes (PDF 4.87 MB) Chapter 10 Plate ...





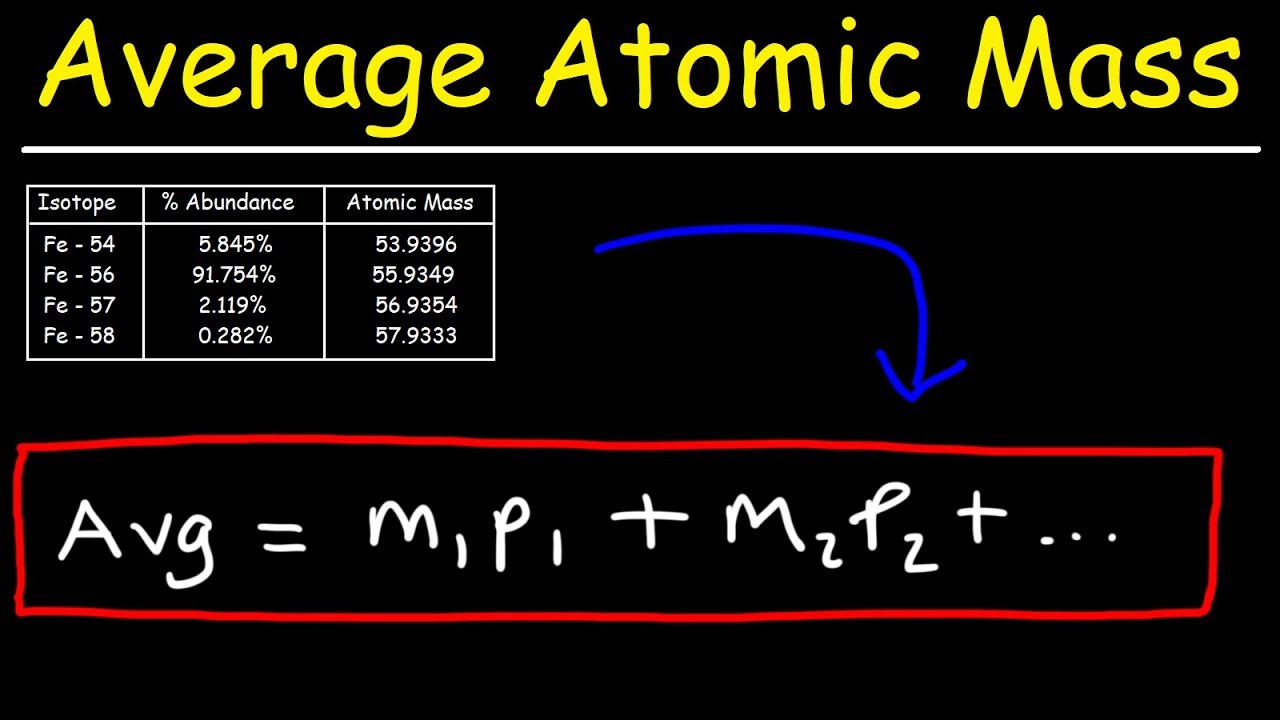





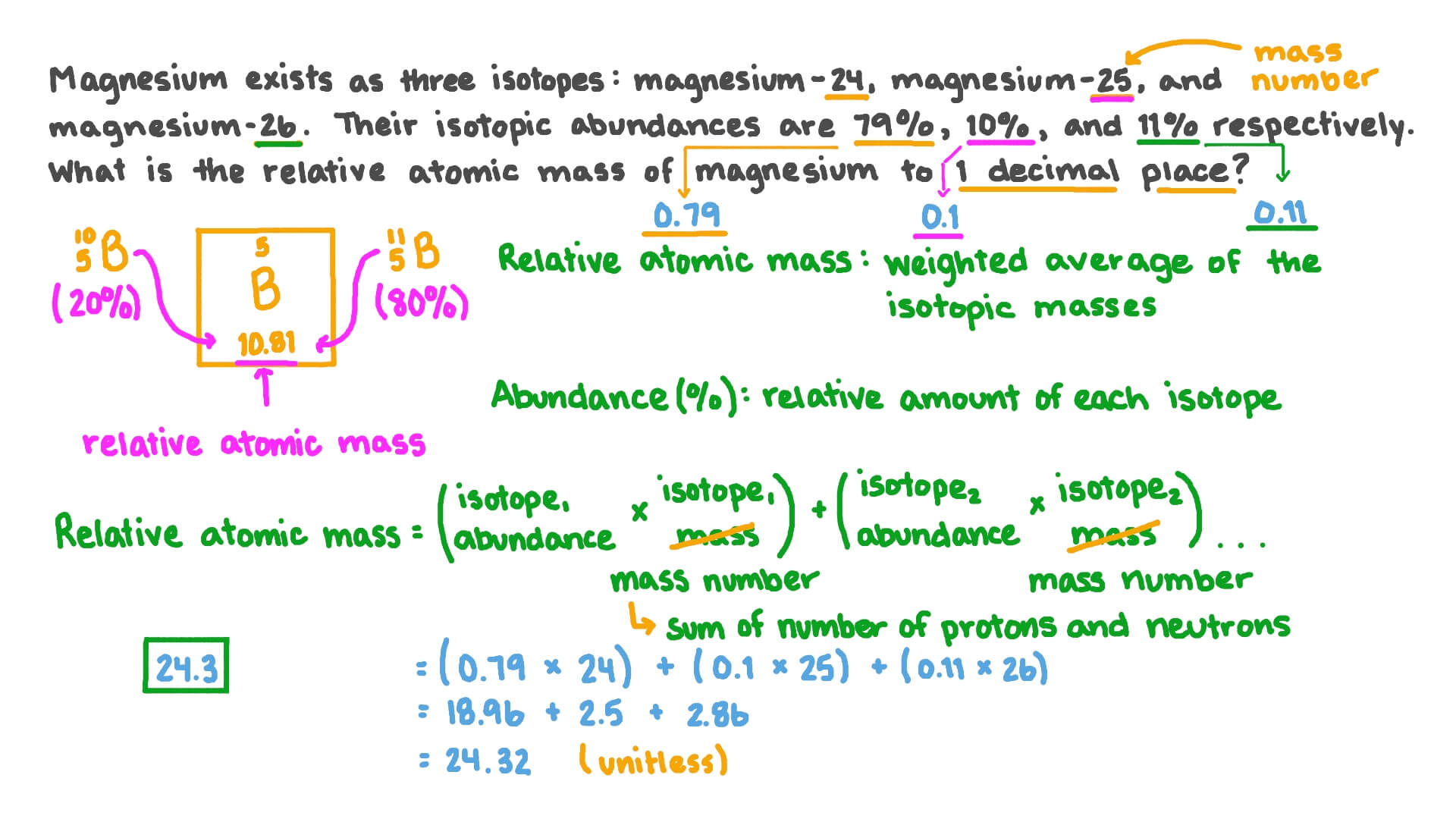





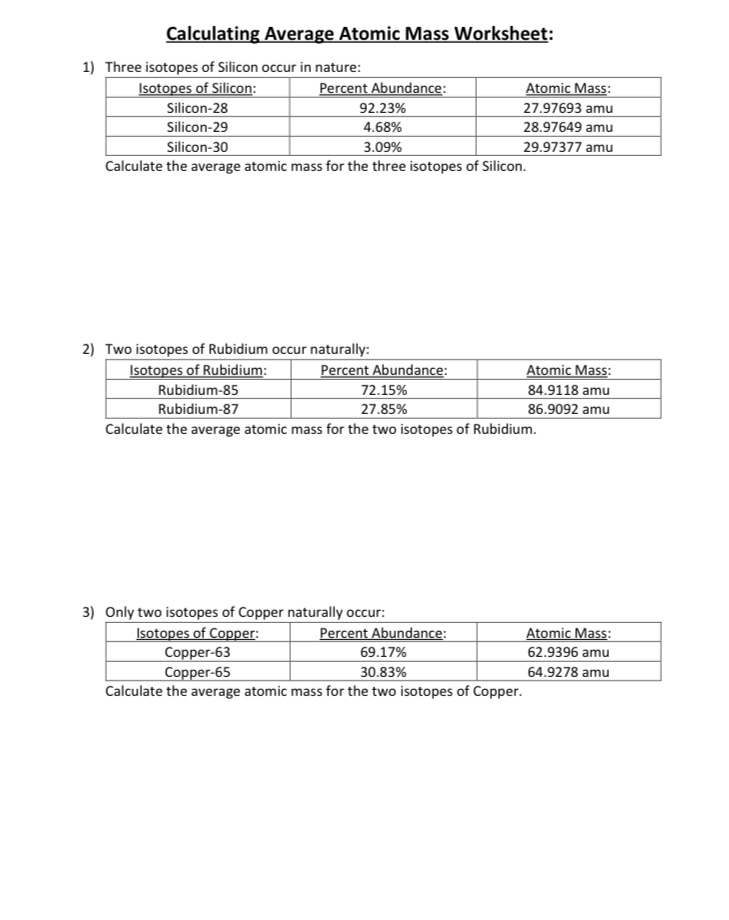










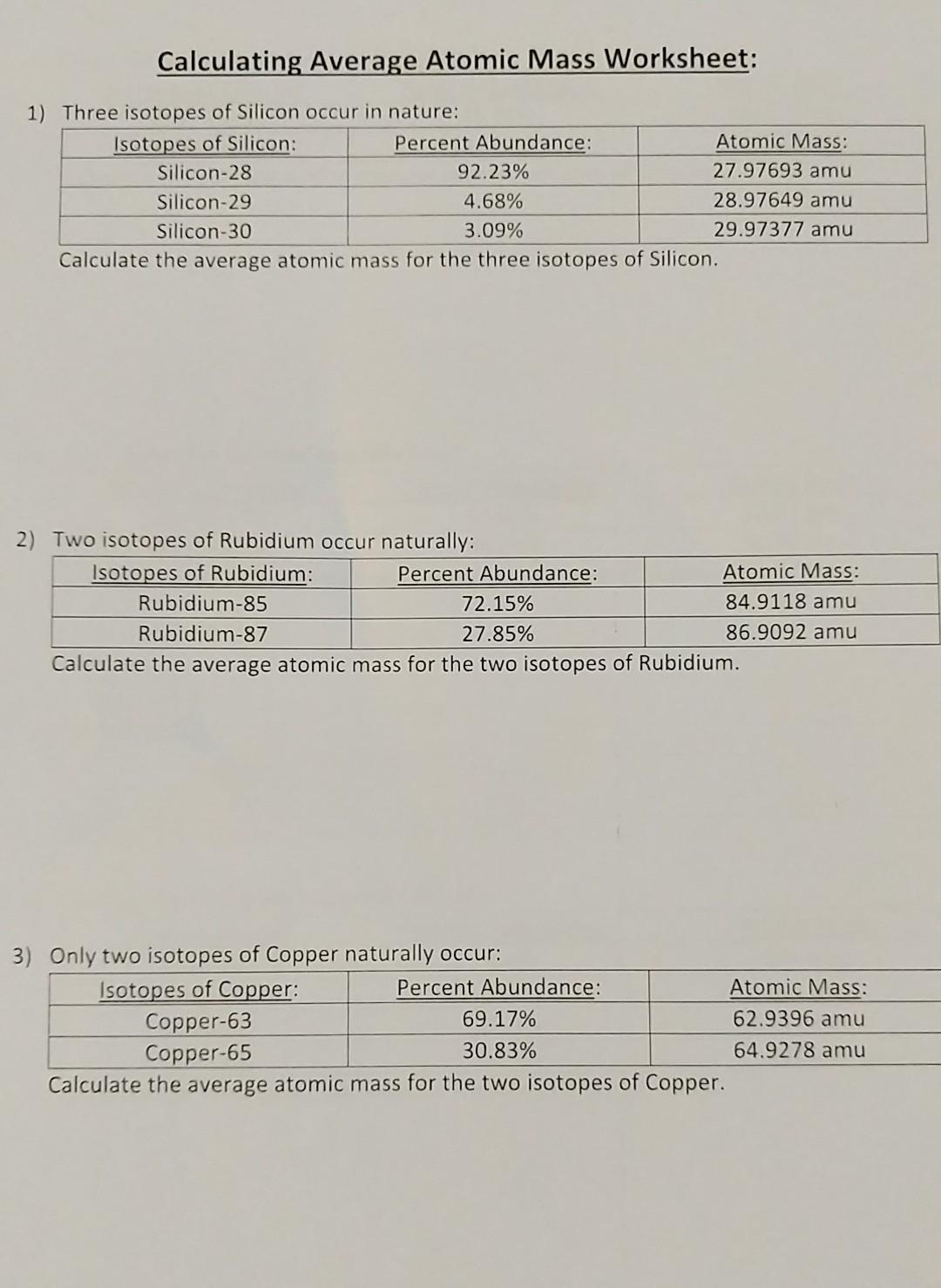

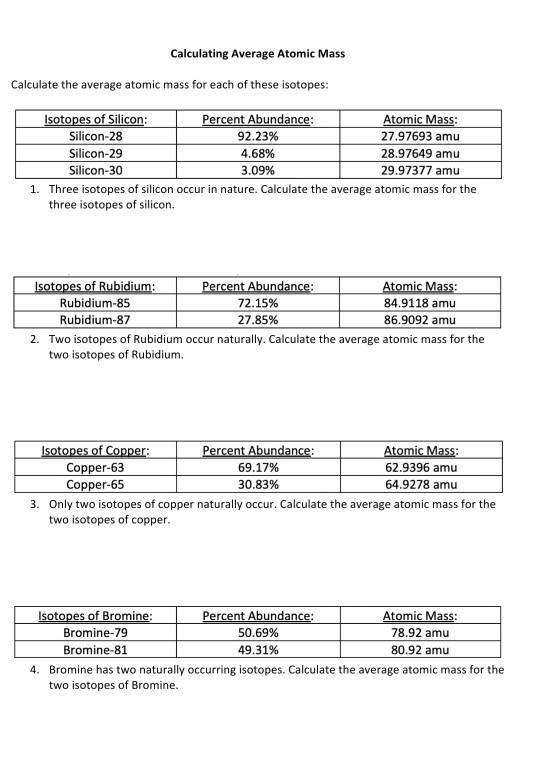





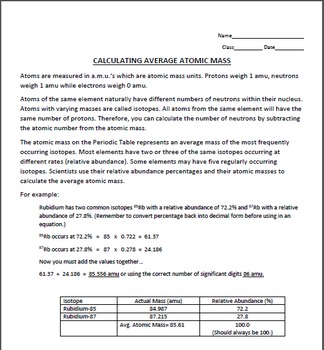

0 Response to "43 more average atomic mass worksheet answers"
Post a Comment