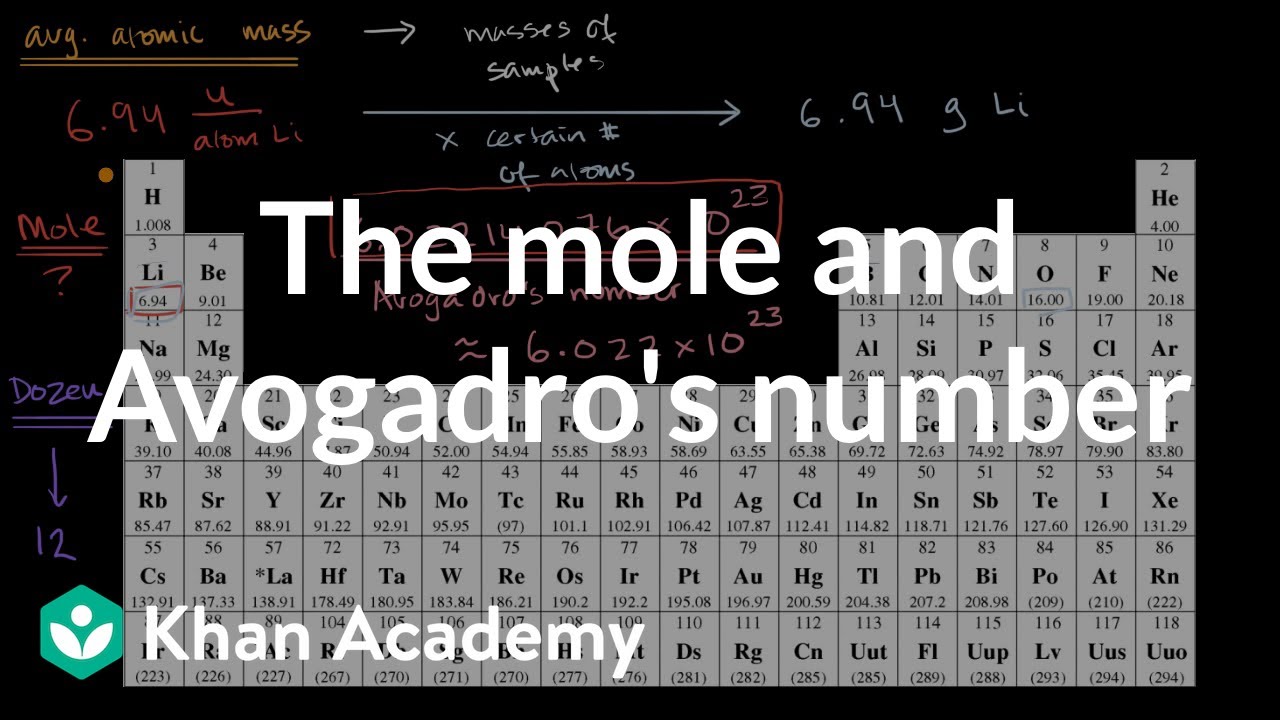
45 the mole and avogadro's number worksheet
study.com › academy › practiceQuiz & Worksheet - Atomic Number and Mass Number | Study.com Quiz & Worksheet - Atomic Number and Mass Number Quiz; Course; Try it risk-free for 30 days Instructions: ... Avogadro's Number: Using the Mole to Count Atoms Quiz; en.wikipedia.org › wiki › GasGas - Wikipedia For his work with gases a century prior, the physical constant that bears his name (the Avogadro constant) is the number of atoms per mole of elemental carbon-12 (6.022 × 10 23 mol −1). This specific number of gas particles, at standard temperature and pressure (ideal gas law) occupies 22.40 liters, which is referred to as the molar volume.
ed.ted.com › lessons › daniel-dulek-how-big-is-aHow big is a mole? (Not the animal, the other one.) - Daniel ... The word “mole” suggests a small, furry burrowing animal to many. But in this lesson, we look at the concept of the mole in chemistry. Learn the incredible magnitude of the mole--and how something so big can help us calculate the tiniest particles in the world.

The mole and avogadro's number worksheet
study.com › skill › learnHow to Use Avogadro's Number to Find the Number of Atoms of a ... Avogadro's number: Avogadro's number, {eq}6.022\times10^{23} {/eq}, is the number of particles in a mole. Mole: Mole is a unit used to count very small things such as atoms or molecules. › topic-1-stoichiometricTopic 1 Stoichiometric relationships - MSJChem - Tutorial ... Mole concept worksheet. ... T he empirical formula and molecular formula of a compound give the simplest ratio and the actual number of atoms ... 1.3 Avogadro's law ... what-if.xkcd.com › 4A Mole of Moles - xkcd It’s also, by chance, a decent ballpark guess for the number of grains of sand on Earth. A mole is also a type of burrowing mammal. There are a handful of types of moles, and some of them are truly horrifying. So what would a mole of moles—602,214,129,000,000,000,000,000 animals—look like? First, let’s start with wild ballpark ...
The mole and avogadro's number worksheet. › test-prep › mcatStoichiometry questions (practice) | Khan Academy The mole and Avogadro's number. Stoichiometry example problem 1. Stoichiometry. Limiting reactant example problem 1 edited. Specific gravity. Next lesson. what-if.xkcd.com › 4A Mole of Moles - xkcd It’s also, by chance, a decent ballpark guess for the number of grains of sand on Earth. A mole is also a type of burrowing mammal. There are a handful of types of moles, and some of them are truly horrifying. So what would a mole of moles—602,214,129,000,000,000,000,000 animals—look like? First, let’s start with wild ballpark ... › topic-1-stoichiometricTopic 1 Stoichiometric relationships - MSJChem - Tutorial ... Mole concept worksheet. ... T he empirical formula and molecular formula of a compound give the simplest ratio and the actual number of atoms ... 1.3 Avogadro's law ... study.com › skill › learnHow to Use Avogadro's Number to Find the Number of Atoms of a ... Avogadro's number: Avogadro's number, {eq}6.022\times10^{23} {/eq}, is the number of particles in a mole. Mole: Mole is a unit used to count very small things such as atoms or molecules.




























0 Response to "45 the mole and avogadro's number worksheet"
Post a Comment