43 calculating average atomic mass worksheet
Calculating Average Atomic Mass Worksheet Calculating Average Atomic Mass Worksheet Name_____ 1. The term "average atomic mass" is a _____average, and so is calculated differently from a "normal" average. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for ... Calculating Average Atomic Mass Practice - Study.com Practice Calculating Average Atomic Mass with practice problems and explanations. Get instant feedback, extra help and step-by-step explanations.
Average Atomic Mass Worksheet Teaching Resources | TPT Practice calculating average atomic mass with this 12 problem worksheet. Perfect for classwork, homework, extra practice, or as examples for students in a distance learning setting.This product includes the following as a PDF file:12 Problems - Calculating Average Atomic MassAnswer Key Downloading this product grants permission for use by one teacher in his or her own classroom.
Calculating average atomic mass worksheet
Isotopes Worksheet - Google Docs 3. Naturally occurring europium (Eu) consists of two isotopes was a mass of 151 and 153. Europium-151 has an abundance of 48.03% and Europium-153 has an abundance of 51.97%. What is the average atomic mass of europium? 4. Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), ... Chemistry Average Atomic Mass Worksheet Answer Chemistry Average Atomic Mass Worksheet Answer October 12, 2022 June 21, 2022 by tamble In this article, you'll learn more about the branches of chemistry, identifying molecules, chemical reactions, and electroneutrality. Average Atomic Mass Worksheet | PDF - Scribd Average Atomic Mass Worksheet - Read online for free. Worksheet for finding average atomic mass.
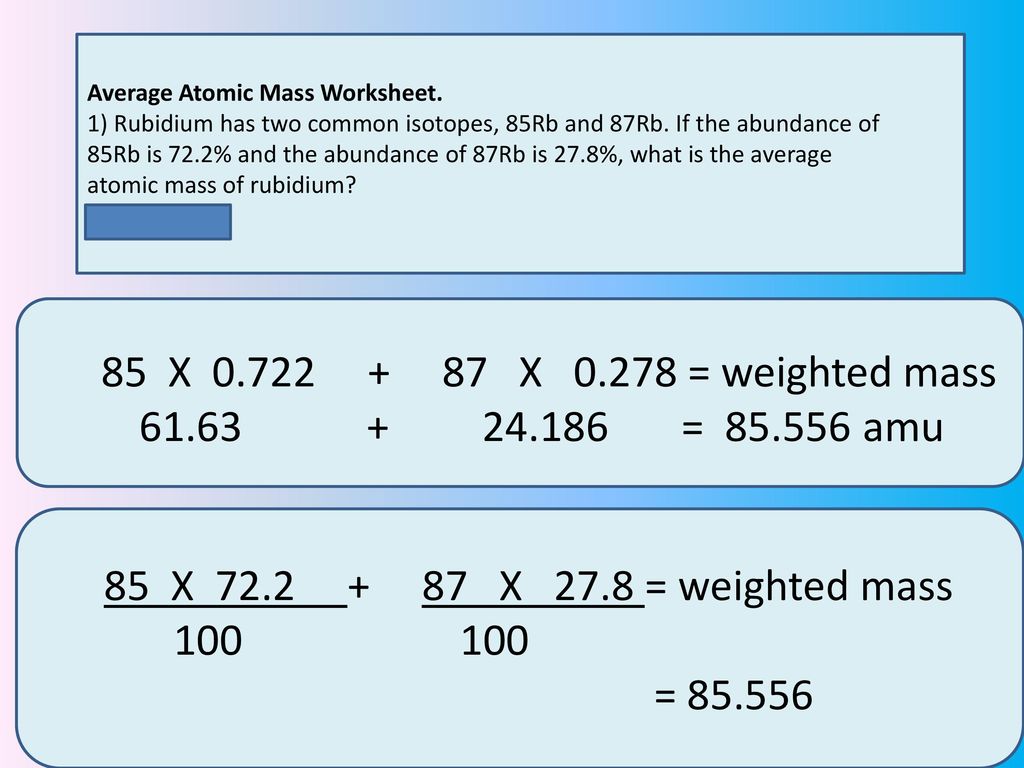
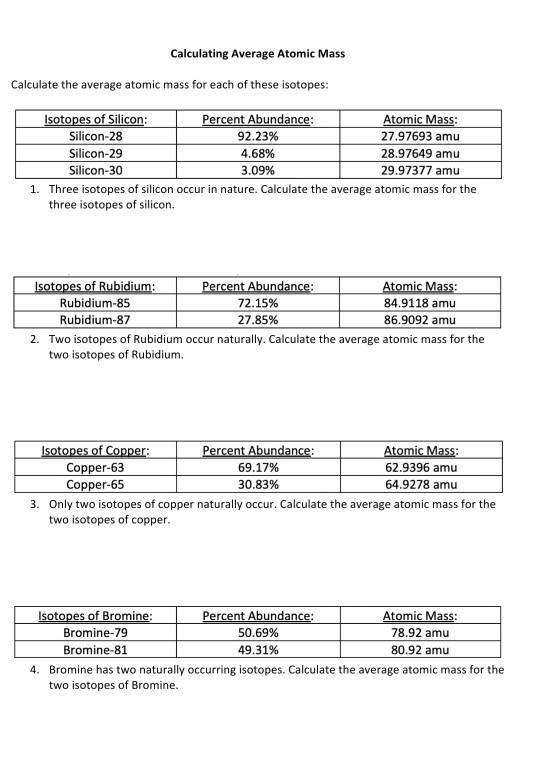
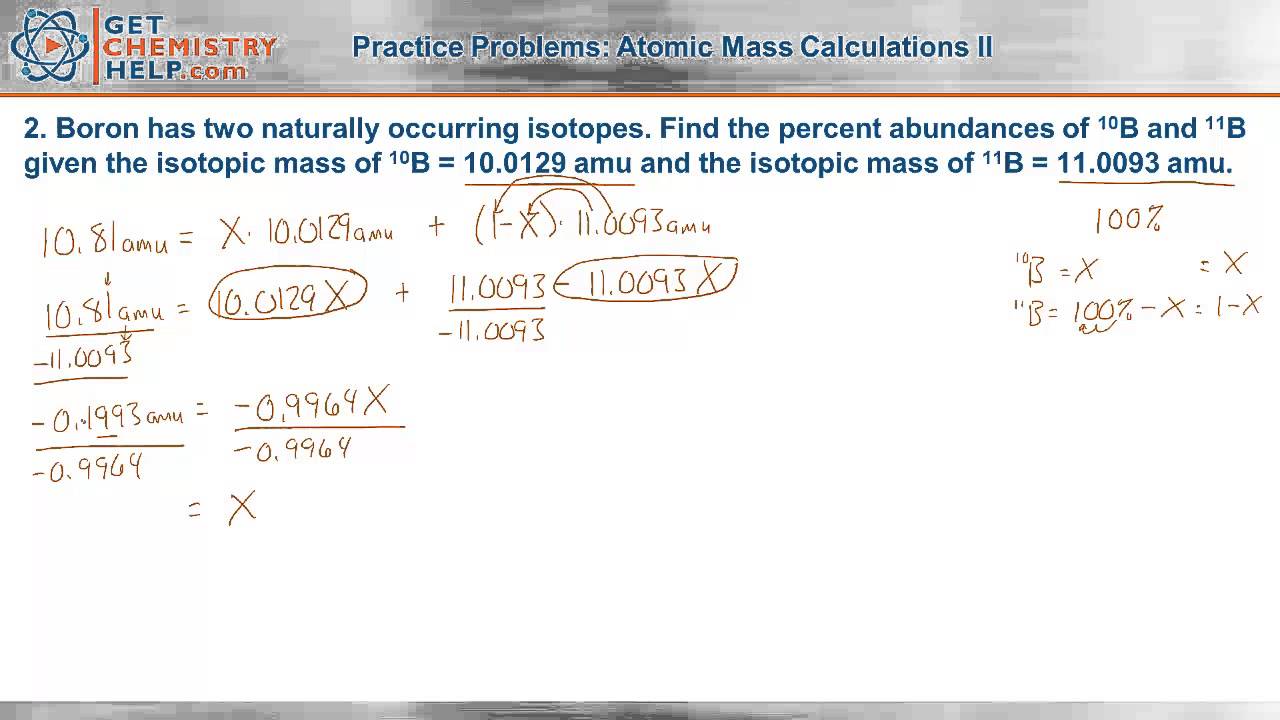
Calculating average atomic mass worksheet. Calculating Atomic Mass Worksheet Answers Chapter 4 Calculating Average Atomic Mass Worksheet Highlight the black box to check your answer. 1. The term "average atomic mass" is a average, and so is calculated differently from a "normal" average. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance are 69.2% and 30.8% respectively. Calculating Average Atomic mass Worksheet-1 ( 1).docx Calculating Average Atomic Mass (2C) Worksheet: 1. Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu Calculate the average atomic mass for the three isotopes of Silicon. Calculating Average Atomic Mass Worksheet Name The relative abundance and atomic masses are: 69.2% for mass of 62.93u. 30.8% fora mass of 64.93u. Calculate the average atomic mass of copper. 2.3: Calculating Atomic Masses (Problems) - Chemistry LibreTexts Average atomic masses listed by IUPAC are based on a study of experimental results. Bromine has two isotopes, 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average atomic mass of Br based on these experiments.
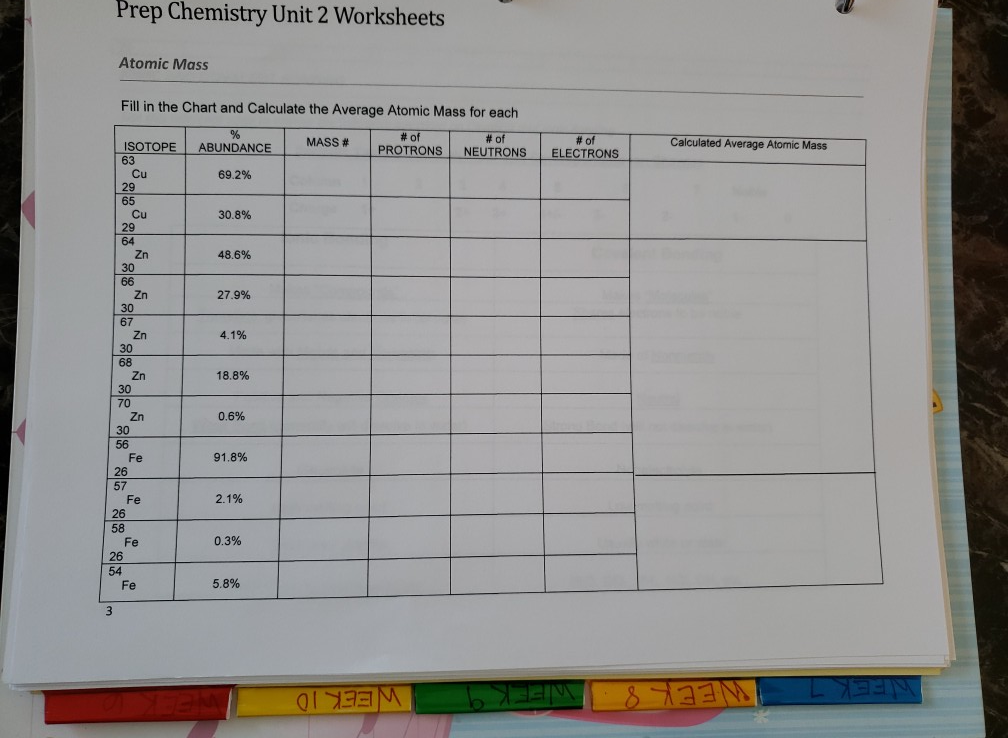
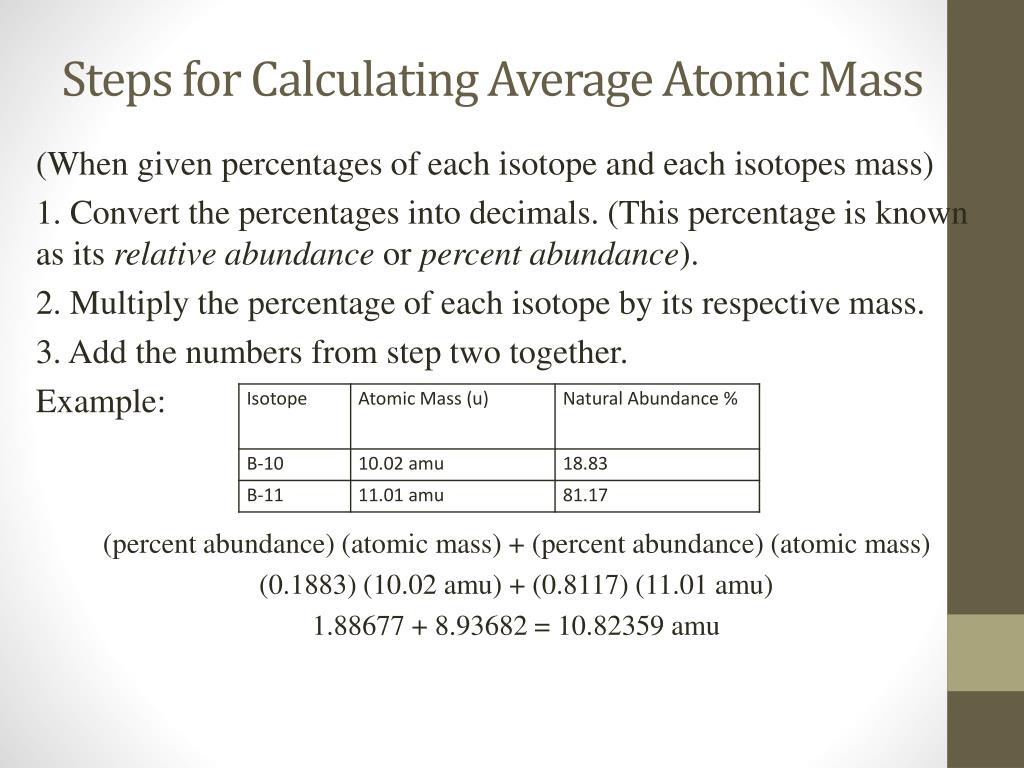
Calculating Average Atomic Mass Worksheet - Name ... - StuDocu Calculating Average Atomic Mass Worksheet University The City College of New York Course Physical Chemistry I (CHEM 33000) Academic year2020/2021 Helpful? 01 Comments Please sign inor registerto post comments. Students also viewed LAB Covalent - Ionic (Part 1) Lewis Structures of Covalent Compounds - 11 Black Chemists PDF Atoms and Isotopes Worksheet Atoms and Isotopes Worksheet Fill in the table with the correct information Isotope Isotope notation Atomic # Protons Electrons neutrons Oxygen-16 8 8 8 8 ... Calculate the average atomic mass of chlorine if its isotopes and % abundance are as follows. Show work. Mass of Isotope % abundance 36.96590 24.47 (.2447)(36.96590) = 9.04556 ... Calculating Average Atomic Mass Worksheet - Peoria Public Schools The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93 amu. Calculate the average atomic mass of copper. 0.692( ... How to Calculate Average Atomic Mass | Chemistry | Study.com How to Calculate Average Atomic Mass Step 1: Identify the percentage of each isotope in the composition of the element and its mass. Step 2: For each isotope, multiply its mass by the...
Calculating Average Atomic Mass Worksheet Name - Scribd Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971amu and 4.22% have a mass of 33.967amu. 4. The four isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus. Average Atomic Mass Calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. Isotope mass (u) relative abundance. Mg-24 23.985 78.70%. Average Atomic Mass Calculations Worksheet Worksheet. Directions: Calculate the average atomic mass for the elements whose isotopes are given below: ... units, and 3.09 % have a mass of 29.974 units. DOC Chemistry Worksheet - Livingston Public Schools Calculate the average atomic masses. Use the atomic mass for significant digits. SHOW ALL WORK FOR CREDIT! What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177.0, 27 have a mass of 178.0, 14 have a mass of 179.0, and 35 have a mass of 180.0? 178.55amu; use three significant digits: 179amu
Calculating Average Atomic Mass Worksheet The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93 amu. Calculate the average atomic mass of copper. 43.54.
How to Calculate Average Atomic Mass. Average atomic mass can be found on the periodic table. Formula to calculate average atomic mass. Example: Consider the chlorine isotopes, chlorine-35 has a mass of 34.969 , while chlorine-37 has a mass of 36.966 amu, if their natural abundance is 75.77% and 24.23% respectively, calculate their average atomic mass. Chlorine - 35 = 34.969 x 0.7577
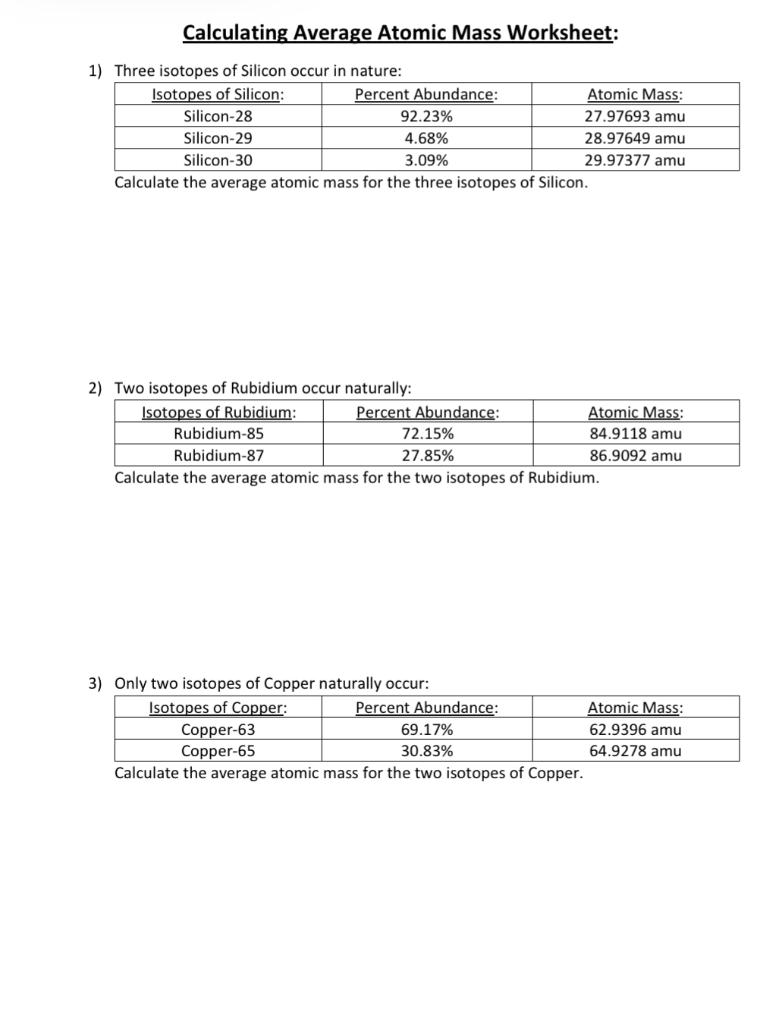
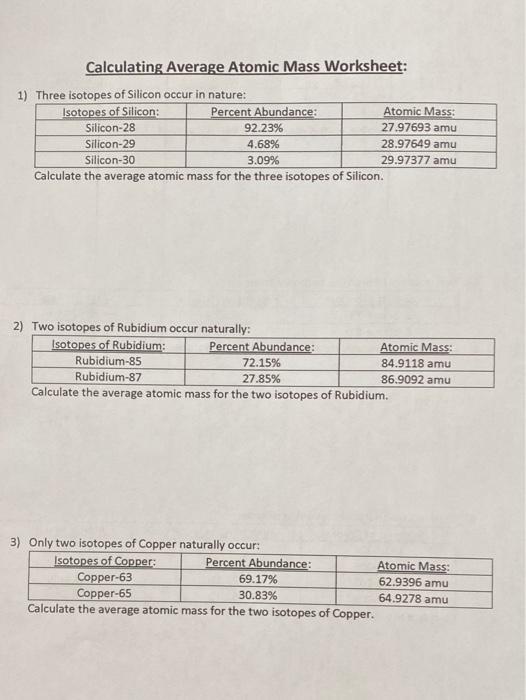
PDF Calculating Average Atomic Mass Worksheet - SI PROGRAM Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Atomic Mass: 27.97693 amu 28.97649 amu 29.97377 amu Isoto es of Silicon: Silicon-28 Silicon-29 Silicon-30 Percent Abundance: 92.23% 4.68% 3.09% Calculate the average atomic mass for the three isotopes of Silicon. (-9 Y(.o ( zq.q 7377) 0/0 2) Two isotopes Of ...
Calculating Average Atomic Mass Worksheet Calculating Average Atomic Mass Worksheet The most necessary spell verify setting is the language , which determines what dictionary Excel uses. Depending on the version of Excel that you're utilizing and the alternatives you made whereas installing the software program, you may be utilizing a quantity of languages during a spell verify operation.
Calculating Average Atomic Mass Worksheet - Name ___Lisa Calculating Average Atomic Mass Worksheet Chemical bonding worksheet Chemical bonding worksheet Other related documents Fall2019-BIO20600-Exam1Review Lab 13 and 14 - Investing for Retirement Activity Forming compounds with Polyatomic Ions Forming compounds with Polyatomic Ions Drawing Branched Alkanes Preview text
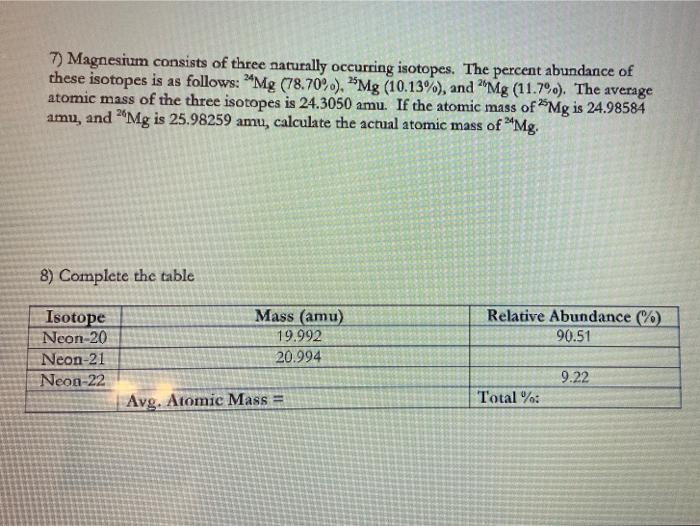
Atomic Mass Worksheet Average Atomic Mass Worksheet: show all work. 1) Rubidium is a soft, ... amu, and 26Mg is 25.98259 amu, calculate the actual atomic mass of 2Mg.
Average Atomic Mass Worksheet: show all work. - studocu.com Practice answering average atomic mass questions average atomic mass worksheet: show all work. rubidium is soft, metal that has two common isotopes, 85rb and ... Calculate the average atomic mass. Copper used in electric wires comes in two flavors (isotopes): 63 Cu and 65 Cu. 63 Cu has an atomic mass of 62 amu and an abundance of 69%. The other ...
PDF NAME Average Atomic Mass Worksheet: show all work. and 24.47 percent 37Cl (mass = 36.966 amu). Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu.
Calculating Average Atomic Mass Worksheet Answers Calculating Average Atomic Mass Worksheet Highlight the black box to check your answer. 1. The term "average atomic mass" is a average, and so is calculated differently from a "normal" average. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance are 69.2% and 30.8% respectively.
Calculating Average Atomic Mass Worksheet Name Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 32, 0.76% has a mass of 33 and 4.22% have a mass of 34. 3. The four isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus.
PDF Average Atomic Mass Practice Problems - scott.kyschools.us 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and
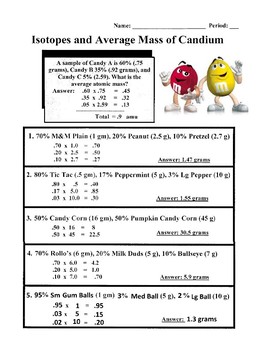
Average Atomic Mass - 2 worksheet Average Atomic Mass - 2 Practice Calculating Average Atomic Mass ID: 3276345 Language: English School subject: Chemistry Grade/level: 10 Age: 15-16 Main content: Atomic Mass Other contents: Average Atomic Mass Add to my workbooks (0) Add to Google Classroom Add to Microsoft Teams Share through Whatsapp Link to this worksheet: Copy jlinde Finish!!
Quiz & Worksheet - Average Atomic Mass | Study.com About This Quiz & Worksheet. Test your understanding of average atomic mass and the steps used to calculate it with this quiz/worksheet combo. All of the questions on these resources are multiple ...
Lesson Worksheet:Percentage Isotopic Abundance | Nagwa In this worksheet, we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic masses. Q1: Chlorine has two stable isotopes, 3 5 C l and 3 7 C l , with atomic masses 34.9689 u and 36.9659 u respectively. The relative abundance of 3 7 C l in an average sample of chlorine is 3 7 3 5 C l C l = 0. 3 1 9 6.
Calculating Average Atomic Mass Worksheet | Aurumscience.com. Calculating Average Atomic Mass Part of understanding isotopes is realizing how their abundance determines the average atomic mass shown with each element of the periodic table. This worksheet will show students how these numbers are calculated, and help them understand why the atomic mass of oxygen is 15.99 AMU instead of simply 16 AMU.
Calculating Average Atomic Mass Worksheet.docx - Name Calculate the average atomic mass of copper. 2.Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% has a mass of 32.971u and 4.22% have a mass of 33.967u. 3. What is the average atomic mass of strontium? Naturally occurring strontium consists of four isotopes, Sr-84, Sr-86, Sr-87 and Sr-88.
Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet Calculate the average atomic mass for each element based on the natural abundance of its isotopes. Terms in this set (4) Find the average atomic mass for Li if 7.5% of Li atoms are 6Li with a mass of 6.0151223 amu and 92.5% are 7Li with a mass of 7.0160041 amu. (7.5 x 6.01251223) + (92.5 x 7.0160041) / 100; 45.0938417 + 648.9803793 / 100 =
Honors Chemistry Calculating Average Atomic Mass Worksheet Answers November 27, 2022 by tamble Honors Chemistry Calculating Average Atomic Mass Worksheet Answers - You're here because you are looking for Chemistry Worksheet Answers. This article will teach you about the different branches of Chemistry, including identifying molecules, chemical reactions and electroneutrality.
Average Atomic Mass Worksheet | PDF - Scribd Average Atomic Mass Worksheet - Read online for free. Worksheet for finding average atomic mass.
Chemistry Average Atomic Mass Worksheet Answer Chemistry Average Atomic Mass Worksheet Answer October 12, 2022 June 21, 2022 by tamble In this article, you'll learn more about the branches of chemistry, identifying molecules, chemical reactions, and electroneutrality.
Isotopes Worksheet - Google Docs 3. Naturally occurring europium (Eu) consists of two isotopes was a mass of 151 and 153. Europium-151 has an abundance of 48.03% and Europium-153 has an abundance of 51.97%. What is the average atomic mass of europium? 4. Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), ...



























0 Response to "43 calculating average atomic mass worksheet"
Post a Comment